Carbon dioxide crystals
Carbon Dioxide Crystals: A Closer Look at Martian Clouds
Carbon dioxide (CO2) crystals in Martian clouds have long fascinated scientists, but their exact shapes and properties are still not fully understood. However, by examining the behavior of CO2 crystals on Earth and making reasonable predictions, we can gain valuable insights into the potential crystal forms found in the Martian atmosphere.
The Symmetry Systems of Crystals
All crystals fall into one of six symmetry systems, each producing a limited number of precise crystal forms. On Earth, water-ice in cirrus clouds (known as Ice Ih) exhibits hexagonal symmetry, resulting in the formation of hexagonal prisms and pyramids. In contrast, solid carbon dioxide possesses cubic symmetry, giving rise to a variety of possible crystal shapes.
Exploring the Lattice Structure
To better understand CO2 crystals, let's imagine ourselves walking around a magnified crystal where individual atoms are visible. We would observe that these atoms align themselves in orderly rows and planes, forming distinct avenues and pathways through the lattice. These pathways correspond to the crystal faces, which determine the overall shape of the crystal. Other directions would lead to increased energy and disorder, rendering them less stable.
Crystal Forms and Habits
Within a cubic symmetry lattice, various crystal forms can arise, including cubes, octahedra, and twelve-sided rhombic-dodecahedra. Additionally, combinations of these forms can occur, resulting in unique shapes such as cuboctahedra. It's important to note that these crystal forms do not necessarily have to be regular; as long as the interfacial angles remain constant, the relative sizes of the faces can change, leading to a variety of habits for each form.
Unveiling CO2 Crystal Structures
Microscopic CO2-ice crystals were first discovered in 1912 and have since been extensively studied. Electron micrographs of laboratory CO2 crystals have revealed the presence of cuboctahedra and octahedra. However, it's crucial to understand that the existence of these forms in the laboratory does not guarantee their occurrence in Martian clouds.
The Potential for Halo Formation
CO2 is transparent at visible wavelengths and exhibits stronger refractive properties compared to water-ice. Armed with these predictions and data, scientists can make accurate forecasts regarding the formation of halos in the Martian sky. The unique crystal structures and refractive nature of CO2 suggest that halo phenomena on Mars may differ from those observed on Earth.
Further Research and Discoveries
While our understanding of carbon dioxide crystals in Martian clouds has improved over time, there is still much to learn. Ongoing research efforts aim to investigate the actual shapes and habits of CO2 crystals in the Martian atmosphere. By studying these crystals and their interactions with light, scientists hope to gain valuable insights into the atmospheric optics of Mars.
In conclusion, carbon dioxide crystals in Martian clouds exhibit cubic symmetry and can take on various crystal forms, including cubes, octahedra, and rhombic-dodecahedra. These crystals may also have platelet-like habits, allowing for the formation of intriguing halo phenomena. While laboratory studies have provided valuable information about CO2 crystal structures, further research is needed to confirm their presence in Martian clouds. By unraveling the mysteries of these crystals, scientists can deepen our understanding of atmospheric optics on both Earth and Mars.
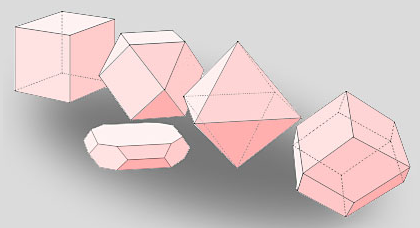
Cubic symmetry crystals. Left to right, cubic, cuboctahedral, octahedral and rhombic dodecahedral forms. The crystals need not be regular provided the interfacial angles are preserved, as in the cuboctahedral platelet habit.
We do not yet know what shapes CO2 crystals actually have in Martian clouds - but we can make reasonable predictions.
All crystals belong to one of only six symmetry systems. Water-ice in Earth's cirrus clouds, Ice Ih, has hexagonal symmetry and forms hexagonal prisms and pyramids. In contrast, solid carbon dioxide has cubic symmetry.
This does not mean that CO2 crystallizes only into microscopic cubes. Imagine walking around an enormously magnified crystal where the individual atoms are visible. They lie in very orderly rows and planes - in several particular directions there are clear avenues and pathways through the lattice. These are the directions taken up by the crystal faces. Facets running in other directions would entail an overall increased energy and disorder and would be less stable. Thus each of the six crystallogrphic symmetry systems in turn produces a limited number of precise crystal forms. A cubic symmetry lattice could give cubic, octahedral, twelve sided rhobic-dodecahedral and other more complicated crystals. Combinations of these forms can occur and so cuboctahedra, solids with six faces having the same direction as a cube and eight more that of an octahedron, might also occur. As with hexagonal water-ice, the crystals need not be regular - provided the interfacial angles stay constant. The relative sizes of the faces can change to produce a variety of habits of each form.
Microscopic CO2-ice was found as long ago as 1912 (ref 1) to have cubic, octahedral and cuboctahedral crystals and recently (ref 2) they have been rephotographed with modern techniques. All these could exist in clouds and rhombic dodecahedral crystals might also exist. Each of these crystal forms might also have flat platelet type habits - imagine the shape being made by machining down the opposite faces of a regular crystal. As with Earth's crystals, the plate habits allow even more fascinating halo possibilities!
Finally, CO2 is transparent at visible wavelengths and is much more strongly refractive than water-ice. Armed with these predictions and data it is quite possible to make accurate predictions of possible halo in Martian skies.
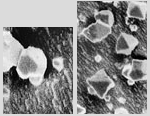
Recent electron micrographs of laboratory CO2 crystals. At top is a cuboctahedron, the lower frame shows several octahedra. These particular crystals are less than a micron across and would not form halos and,of course, existence in the laboratory is no proof that these forms occur in clouds!
Ref. 1 (a) H.E. Behnken, Phys. Rev. 35, 66-73 (1912)
(b) W. Wahl, Z. Physik. Chem. 88, 129-171 (1914)
Ref. 2 (a) Wergin, W. P., J. L. Foster, A. T. C. Chang, D. K. Hall, A. Rango and E. F.Erbe. 1997. Structure of carbon dioxide crystals (Martian snow) as observed in TEM replicas and low temperature SEM images. Microsc. and Microanalysis. (suppl 2): 1235-36.
(b) Foster, J. L., W. P. Wergin, E. Erbe, A. T. C. Chang, and D. K. Hall. 1997.Observations and comparisons of H2O (snow) and CO2 crystals using low-temperature scanning electron microscopy. Workshop on remote sensing of planetary ices: Earth and other solid bodies. Flagstaff, AZ June.
Note: this article has been automatically converted from the old site and may not appear as intended. You can find the original article here.
Reference Atmospheric Optics
If you use any of the definitions, information, or data presented on Atmospheric Optics, please copy the link or reference below to properly credit us as the reference source. Thank you!
-
<a href="https://atoptics.co.uk/blog/carbon-dioxide-crystals/">Carbon dioxide crystals</a>
-
"Carbon dioxide crystals". Atmospheric Optics. Accessed on April 23, 2024. https://atoptics.co.uk/blog/carbon-dioxide-crystals/.
-
"Carbon dioxide crystals". Atmospheric Optics, https://atoptics.co.uk/blog/carbon-dioxide-crystals/. Accessed 23 April, 2024
-
Carbon dioxide crystals. Atmospheric Optics. Retrieved from https://atoptics.co.uk/blog/carbon-dioxide-crystals/.